 VOLUME 26, ISSUE 2 • June 2022. Full issue »
VOLUME 26, ISSUE 2 • June 2022. Full issue »

Application of Misfolded Protein Amplification Assays for Early Differentiation of Neurodegenerative Parkinsonisms
Synucleinopathies define a spectrum of neurodegenerative diseases, including multiple system atrophy (MSA) and Parkinson’s disease (PD), associated with the misfolding and aggregation of α-synuclein (αSyn)1. Clinically, it is challenging to differentiate PD and MSA, especially in the early stages of disease, and antemortem misdiagnosis between these two diseases is common2.
In addition, differentiating synucleinopathies, particularly MSA, from other neurodegenerative proteinopathies presenting with parkinsonism (such as tau proteinopathies) poses a diagnostic challenge for the clinical practice. In recent years, seed amplification assays (SAAs) have emerged as a reliable method of detecting minute amounts of misfolded disease-associated proteins such as prion protein, tau and αSyn3. These assays, also known as Real-Time Quaking-induced Conversion (RT-QuIC) and protein misfolding cyclic amplification (PMCA), are able to reproduce in vitro the process of protein misfolding and accumulation that occur in the brain, taking advantage on the ability of these proteins to self-propagate by amplifying the small amounts of misfolded protein that are present in biological fluids at the expense of recombinant monomeric protein included in the assay4,5. This process is monitored in real-time due to the dye thioflavin T, that emits fluorescence when a new aggregate is generated (Figure 1a).
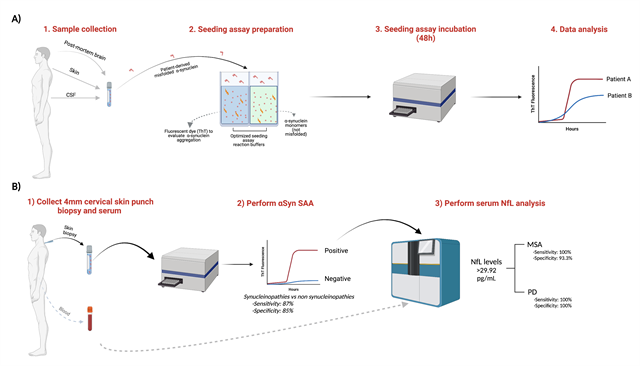
Figure 1:
A) Schematic of the α-synuclein seeding assay (α-Syn SAA) to evaluate the presence of misfolded α-synuclein in a variety of biofluids. B) Workflow for the identification of individuals with MSA and PD, with high sensitivity and specificity, from amongst a mixed population of individuals representative of those typically referred for assessment for a suspected parkinsonian disorder. Figure modified from Martinez-Valbuena, I. et al12 and created with Biorender.com.
However, and despite the reliability of these assays to consistently detect αSyn seeds in PD using a variety of biological samples 6-9, one of the major challenges in achieving early differentiation of neurodegenerative parkinsonisms has been the inconsistent detection of disease-associated αSyn in MSA patients using SAAs. Although the reason for this is not yet fully understood, several studies have demonstrated that the selection of the right conditions to conduct the amplification of the aggregates is critical to the outcome10. Thus, we sought to identify the optimal assay conditions that favor MSA seeding activity, by systematically modulating the ionic and cationic compositions and the pH of the αSyn SAA reaction buffer11. Our study of 168 conditions, has proven that the physicochemical factors that govern the in vitro amplification of αSyn can be tailored to generate strain-specific reaction buffers for use in αSyn SAA.
By applying two carefully selected buffer conditions we were able to differentiate αSyn-derived from post-mortem brain samples of subjects with a neuropathological diagnosis of PD, MSA, progressive supranuclear palsy (PSP) and control subjects, with great sensitivity and specificity. Furthermore, to assess whether these findings could be translated into clinical practice, in collaboration with Dr. Anthony Lang, Toronto Western Hospital, we employed this novel assay to assess the presence of misfolded αSyn in the periphery using a single 4-mm cervical skin biopsy from 50 clinically diagnosed patients with MSA, PD, PSP and healthy controls12.
Our analysis showed that cutaneous αSyn SAA achieved a combined sensitivity of 87% and specificity of 85% for synucleinopathies vs non-synucleinopathies. Furthermore, combining the αSyn SAA with the concentration of serum neurofilament light chain (NfL), allowed the distinction between MSA and PD patients with very high specificity and a sensitivity. Circulating NfL was significantly higher in patients with MSA than in patients with PD (p=0.0004), and not significantly different between controls and PD patients or between PSP and MSA patients. Thus, a positive skin αSyn SAA was highly sensitive and specific for a synucleinopathy and the second step NfL assay differentiated MSA from PD (Figure 1b). These promising preliminary results will require validation in independent cohorts and further exploration before our approach can be widely applied. However, the future application of such a non-invasive 2-step diagnostic approach in the screening process for clinical trials has the potential to ensure that trials for therapeutics targeting αSyn recruit individuals with a positive cutaneous αSyn SAA (and distinguish MSA from PD as necessary).
Share this article:
Tweet
References:
- Kovacs, G. G. Molecular pathology of neurodegenerative diseases: Principles and practice. J. Clin. Pathol. 72, 725–735 (2019).
- Di Luca, D. G. & Lang, A. E. Is clinical assessment enough? Moving toward early differentiation of neurodegenerative parkinsonisms. Brain 177, (2021).
- Ferreira, N. do C. & Caughey, B. Proteopathic Seed Amplification Assays for Neurodegenerative Disorders. Clin. Lab. Med. 40, 257–270 (2020).
- Schmitz, M. et al. The real-Time quaking-induced conversion assay for detection of human prion disease and study of other protein misfolding diseases. Nat. Protoc. 11, 2233–2242 (2016).
- Bongianni, M. et al. Diagnosis of human prion disease using real-time quaking-induced conversion testing of olfactory mucosa and cerebrospinal fluid samples. JAMA Neurol. 74, 155–162 (2017).
- Manne, S. et al. α-Synuclein real-time quaking-induced conversion in the submandibular glands of Parkinson’s disease patients. Mov. Disord. 35, 268–278 (2020).
- Wang, Z. et al. Skin α-Synuclein Aggregation Seeding Activity as a Novel Biomarker for Parkinson Disease. JAMA Neurol. 78, 30 (2021).
- van Rumund, A. et al. Alpha-synuclein RT-QuIC in the CSF of uncertain cases of parkinsonism. Ann. Neurol. (2019). doi:10.1002/ana.25447
- Rossi, M. et al. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol. 140, 49–62 (2020).
- Metrick, M. A. et al. Million-fold sensitivity enhancement in proteopathic seed amplification assays for biospecimens by Hofmeister ion comparisons. Proc. Natl. Acad. Sci. U. S. A. 116, 23029–23039 (2019).
- Martinez-Valbuena, I. et al. Alpha-synuclein seeding shows a wide heterogeneity in multiple system atrophy. Transl. Neurodegener. 11, 7 (2022).
- Martinez-Valbuena, I. et al. Combining Skin α-Synuclein Real-Time Quaking-Induced Conversion and Circulating Neurofilament Light Chain to Distinguish Multiple System Atrophy and Parkinson’s Disease. Mov. Disord. 37, 648–650 (2022).
Read more Moving Along:






