 VOLUME 29, ISSUE 2 • JUNE 2025. Full issue »
VOLUME 29, ISSUE 2 • JUNE 2025. Full issue »

Digital outcomes as biomarkers of disease progression in early Parkinson’s disease: A systematic review
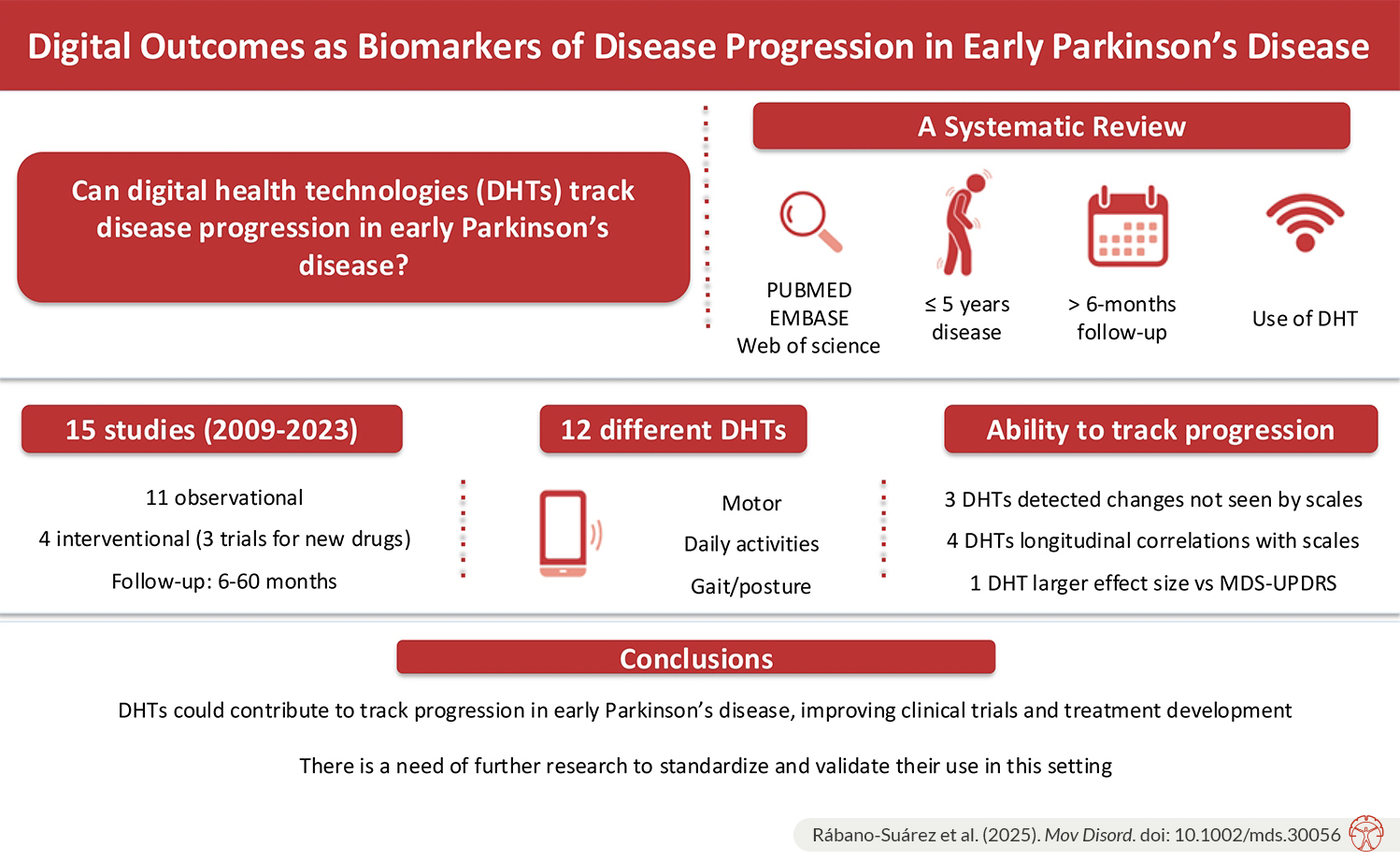
Outcomes derived from digital health technologies (DHTs) are emerging as promising biomarkers in Parkinson’s disease (PD), particularly for monitoring disease progression and potentially reducing sample sizes in clinical trials. Several studies in PD have already incorporated exploratory digital outcomes alongside traditional scales. However, a clear regulatory framework and consensus on their implementation remain lacking. The objective of this study was to focus on early PD, the most targeted stage in neuroprotective clinical trials. We specifically examined DHTs in terms of concurrent validity and sensitivity to change.
We included studies that met specific inclusion criteria: early-stage PD patients (disease duration ≤5 years), longitudinal assessments using body-worn sensors, mobile apps, or activity trackers, and a follow-up period of at least six months.
A total of 15 studies were included after screening, with most published in the past five years. Eleven were observational studies, while three were clinical trials investigating new drugs. Sample sizes were predominantly small, mostly in the double-digit range.
The selected studies employed 12 different DHTs, highlighting significant technological heterogeneity. Four studies used passive monitoring, eight incorporated active monitoring, and eight included in-hospital tests. DHTs captured digital features related to motor function and daily activities, with five studies focusing on axial measures. All but one study compared DHT-derived metrics with clinical rating scales to measure clinical progression, primarily the UPDRS, MDS-UPDRS (especially part III), or composite scores derived from them.
Some studies observed that DHTs captured progression in parallel with traditional scales. In terms of digital outcome progression, Pagano et al. detected an annual increase in the PASADENA Digital Motor Score mirroring MDS-UPDRS III. Many studies identified digital biomarkers related to gait and stance that showed progression (Hobert et al., Micó-Amigo et al., Salarian et al.)
Three digital health technologies (DHTs)—used in studies by Brzezicki et al., Mancini et al., and Mirelman et al.—detected longitudinal changes that were not captured by clinical scales. Notably, one study (Mirelman et al.) using the Axivity device—a small, back-worn wearable that passively monitors movement over seven days without requiring patient interaction—reported a larger effect size for digital measures over time compared to the MDS-UPDRS.
Some studies investigated the correlation between clinical progression and digital features, but some focused on cross-sectional correlations at different time points. Four studies (Micó-Amigo et al., Di Lazzaro et al., Goetz et al., Mirelman et al.) reported longitudinal correlations between DHTs and clinical scales.
Despite these promising findings, heterogeneity in study methodologies, sample sizes, and reported outcomes complicates the standardization of DHTs in clinical trials. While DHTs show potential for detecting subtle clinical changes in PD, their correlation with traditional scales varies. Some findings suggest that DHTs may capture complementary aspects of disease progression, but further research is needed to validate their use in clinical trials.
Of note, as for all endpoints, it should be taken into account that it is not enough to show statistical changes in an outcome; it is crucial to also demonstrate that these changes have meaningful consequences for patients. This should be applicable also to DHTs outcomes. Meaningfulness may refer to changes that are noticeable (perceptible to patients or their caregivers) and/or valuable (worthwhile given the costs, inconvenience, or side effects of achieving them).
Finally, to facilitate broader adoption, future studies should employ adequately powered sample sizes, assess multiple clinical dimensions, and ensure the validity and reliability of digital measures. Collaborative efforts, such as MOBILISE-D and IDEA-FAST, are advancing the field by working toward standardized validation and regulatory approval. With further refinement, DHTs could significantly contribute to improving PD clinical trials and treatment development.
References
1. Rábano-Suárez P, Del Campo N, Benatru I, Moreau C, Desjardins C, Sánchez-Ferro Á, Fabbri M. Digital Outcomes as Biomarkers of Disease Progression in Early Parkinson's Disease: A Systematic Review. Mov Disord. 2025 Feb;40(2):184-203. doi: 10.1002/mds.30056. Epub 2024 Nov 29. PMID: 39613480; PMCID: PMC11832816.
Read more Moving Along:






