 VOLUME 26, ISSUE 4 • DECEMBER 2022 Full issue »
VOLUME 26, ISSUE 4 • DECEMBER 2022 Full issue »

Junior Research Award:
Multimodal mechanistic disease stratification in sporadic Parkinson’s disease
Despite multiple trials, there are no disease-modifying therapies available for Parkinson’s. This could be due to the inherent pathogenic heterogeneity of Parkinson’s, with multiple mechanisms implicated in the pathogenesis, such as mitochondrial and lysosomal dysfunction. For future precision medicine approaches to succeed, new tools need to be developed to stratify people with Parkinson’s according to their specific disease-causing pathogenic mechanism.
The group of my supervisor Prof Bandmann had previously demonstrated the utility of phenotyping peripheral tissue in Parkinson’s and identified clear subgroups of participants with evidence of mitochondrial or lysosomal dysfunction. However, peripheral tissue phenotyping is labour intensive, expensive, and limited to the assessment of non-neuronal tissue in vitro.
31Phosphorus magnetic resonance spectroscopy (31P-MRS) offers a non-invasive tool to assess brain bioenergetics and therefore potentially identify those with evidence of mitochondrial dysfunction. Previous 31P-MRS work has found bioenergetic abnormalities in Parkinson’s, however, the pathogenic correlate of these findings is unclear. The aims of this study were to assess the bioenergetics of the brain in vivo using 31P-MRS and in tandem to assess the peripheral tissue for mitochondrial and lysosomal abnormalities in vitro, to determine the pathogenic correlates of the in vivo 31P-MRS bioenergetic abnormalities.
In total, we recruited 60 participants (35 people with Parkinson’s and 25 age- and sex-matched controls) who all underwent 31P-MRS and a skin biopsy. Our 31P-MRS acquisition used a multi-voxel technique (2D chemical shift imaging) to capture spectroscopic signal from both the putamen and the midbrain (capturing substantia nigra within the voxel). These spectra can be analysed to derive relative quantities of key bioenergetic metabolites, such as ATP and inorganic phosphate, to infer the bioenergetic status of the brain tissue. Skin biopsies were also obtained to establish fibroblast cell lines to characterise their mitochondrial and lysosomal phenotype using high-content live cell imaging (see figure) to assess intracellular ATP, mitochondrial membrane potential (MMP), and both the number and morphology of mitochondria and lysosomes.
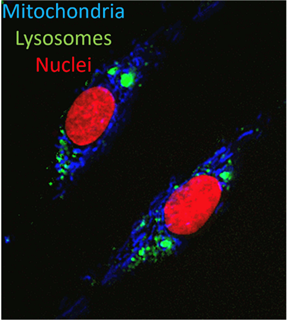
In people with Parkinson’s, we found that lower 31P-MRS putaminal ATP correlated with lower MMP and greater numbers of both mitochondria and lysosomes in the peripheral tissue. In contrast, in healthy controls higher 31P-MRS putaminal ATP correlated positively with higher mitochondrial counts in peripheral tissue, as would be expected. The inverse correlations between 31P-MRS putaminal ATP and both lysosome count and MMP in peripheral tissue (as observed in people with Parkinson’s) was absent in healthy controls. These relationships were not observed between the midbrain and the peripheral tissue.
The observation of lower 31P-MRS putaminal ATP correlating with an increased number of small, dysfunctional mitochondria and accumulation of lysosomes in peripheral tissue would be in keeping with increased mitophagy. The contrast between midbrain and putamen may offer further evidence to support the hypothesis of a prominent link between impaired mitophagy and impaired striatal energy homeostasis as a key event in early Parkinson’s.
This is the first study to mechanistically stratify PD by combining in vivo measures of bioenergetic dysfunction in the brain with relevant pathogenic mechanisms in peripheral tissue. This may also facilitate the stratification of people with Parkinson’s for future precision medicine approaches, particularly for trials targeting mitophagy.
Our future work will focus on further characterising these pathogenic correlates by directly reprogramming peripheral tissue fibroblasts into neurons (see figure), so that both the mitochondrial and lysosomal abnormalities identified can be further characterised in patient derived neuronal tissue.
Read more Moving Along:






