 VOLUME 27, ISSUE 3 • October, 2023. Full issue »
VOLUME 27, ISSUE 3 • October, 2023. Full issue »

Recent breakthroughs: An overview of top findings from the past year
Revisit the takeaways the popular 2023 highlights session: New genetic findings, clinical and biomarker studies, emerging therapeutic targets, and more.
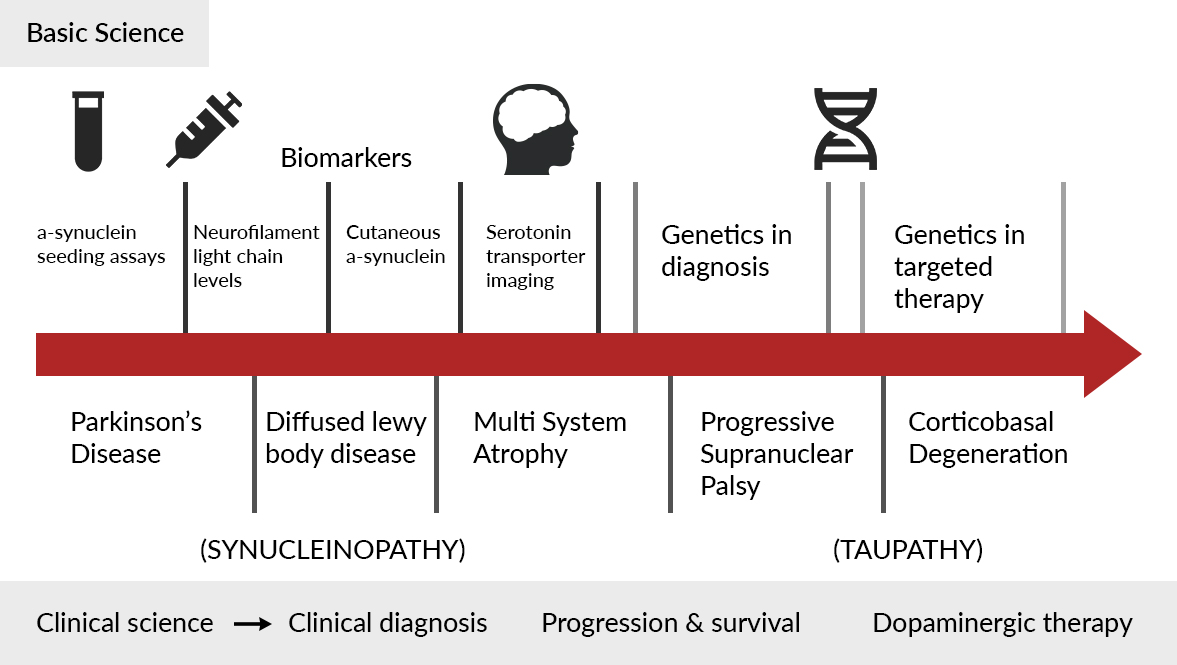
Legend for figure 1: Highlights of 2022-2023 in Hypokinetic Movement Disorders
Hypokinetic highlights
I was invited to present a talk during the MDS International Congress as part of the highlight session, where I presented a summary of hypokinetic movement disorders in the last year.
My presentation was primarily based on a PubMed search using different keywords related to hypokinetic disorders including Parkinson’s disease (PD), Progressive supranuclear palsy (PSP), multiple system atrophy (MSA), parkinsonism and atypical parkinsonism. I also reviewed the bibliography and cross-references of these publications.
Overall, I reviewed more than 10,900 original papers and abstracts, including those accepted for presentation during the MDS International Congress.
I reviewed the publications related to basic and clinical sciences (figure 1). In basic science, the majority of the publications were related to biomarkers. The most common biomarkers were based on CSF and alpha-synuclein seeding assays.1 Also, there has been rapid advancement in blood and saliva-based biomarkers.2-5 Many studies were also related to cutaneous biomarkers and neuroimaging. Studies using brain MRI in PSP with Richardson’s syndrome and variant phenotypes, serotonin transporter imaging in MSA and PD, and Vagus nerve ultrasonography distinguishing MSA from other Parkinsonian syndromes were discussed in detail.6,7 There was an interesting article showing how PSP tauopathy may spread in the brain from cell to cell in a ‘prion-like manner.’8
Some important studies related to genetics-based targeted therapy were also covered. In a phase 2 trial, Venglustat (MOVES-PD) had a satisfactory safety profile but showed no beneficial treatment effect compared with placebo in GBA1-associated Parkinson's disease.9 In another study, LRRK2 inhibition by BIIB122 in healthy participants (phase 1) and patients with PD (phase 1b) were tested.10 At generally safe and well-tolerated doses, BIIB122 achieved substantial peripheral LRRK2 kinase inhibition and modulation of lysosomal pathways downstream of LRRK2, with evidence of CNS distribution and target inhibition. These studies support continued investigation of LRRK2 inhibition with BIIB122 for the treatment of PD.
The phase III results of RISE-PD were published in August 2023, indicating that there was a statistically significant improvement in good on-time per day for IPX203 (extended-release) compared with IR CD-LD (immediate release).11
One of the articles in the clinical section has highlighted that the clinical diagnosis of PD and parkinsonism has improved. In the early stage (<5 years), the accuracy of clinical diagnosis was 84.3% and expert clinical diagnosis was 91.5%. For the final clinical diagnosis, the accuracy further improved to 90.3%, and for the expert clinical diagnosis, it improved to 96.7%.12 The bottom line of the study was that the clinical diagnosis in the right hands is very accurate, and we are getting better.
Two of the recently published articles focused on the progression and survival of PD and Parkinsonism.13,14 In the first study, the progression of clinical markers in prodromal PD and DLB were evaluated.13 It was observed that the motor variables tended to progress faster and required the lowest sample size. In contrast, cognitive, autonomic, and olfactory variables showed modest progression resulting in high sample sizes.
In another study (PROSPECT-M-UK) showing the progression of atypical parkinsonian syndromes, neuroimaging metrics were enabling lower sample sizes to achieve equivalent power for clinical trials than cognitive and functional measures.14 In a recently published study, poorer survival for mutations linked to more aggressive forms (SNCA and GBA), and longer survival for those associated with a more benign disease course (PRKN and LRRK2) was observed.15 In another article, a prognostic model has been developed to predict the overall survival of MSA.16
After the COVID epidemic, zoom meetings have been used for the clinical assessment of patients with parkinsonism, but there have been concerns regarding the accuracy of the data. In a study, the authors have highlighted the potential pitfalls of remote and automated video assessments of movement disorders.17 They have cautioned that the variability in video acquisition should be carefully addressed in future studies of video-based digital biomarkers for PD, particularly if data are acquired remotely.
Overall, I enjoyed presenting the highlights of hypokinetic disorders.
Hyperkinetic highlights
Among recent discoveries in the clinical and basic science literature from the last 12 months in the field of hyperkinetic movement disorders, here are some highlights with important clinical and disease-mechanism implications.
New genetic findings (including the new dystonia-related genes EIF4A2 and ATP5F1B and adult-onset forms of dystonia due to pathogenic variants in the KMT2B and GNAO1 genes) supported the possible continuum between neurodevelopmental disorders and dystonia (Harrer et al., 2023; Nasca et al., 2023; Monfrini et al., 2022; Wirth et al., 2022; Krenn et al., 2022). This concept has important implications for patient counseling and encourages exploring the modulatory role of genetic and environmental traits on the phenotypical variability of monogenic forms of dystonia (Dzinovic et al., 2022: Di Fonzo et al., 2023; Erro et al., 2022).
Broader genotyping and in-depth imaging characterization has also helped detect underlying genetic etiologies in a growing number of patients previously diagnosed with cerebral palsy (CP) (Tsagkaris et al., 2023; Pavelekova et al., 2023).
Concomitantly, more comprehensive genetic approaches continue to be crucial for new gene discovery. Pellerin et al (2023) identified a new intronic expansion in the FGF14 gene that could explain between 10% and 61% of cases of late-onset cerebellar ataxia, a condition that often remains undiagnosed (Pellerin et al., 2023; Wilke et al., 2023). Similarly, new re-arrangements in intronic regions of previously described genes are helping fill the gap of the missing hereditability of cases of Familial Adult Myoclonus Epilepsy (Maroilley et al., 2023).
In already well established monogenic hyperkinetic movements disorders, including Huntington disease (HD) and the spinocerebellar ataxia (SCA), preclinical and biomarker studies are supporting the role of very early intervention, as these conditions are probably characterized by developmental and compensatory changes starting long before the onset of motor symptoms (Braz et al., 2022; Schultz et al., 2023; Maas et al., 2023; Coarelli et al., 2023). Unfortunately, promising therapeutic approaches for HD (i.e. anti SEMA4D antibodies) have not provided the expected results, although secondary outcome measures may suggest a role for treating subgroups of patients (Feigin et al., 2023).
After last year’s successful clinical trial with the calcium channel blocker PRAX-944 in essential tremor (ET), new studies have showed the role of RyR1 in animal models and autoptic tissues of ET patients in causing a pathogenic “leak” of calcium in the endoplasmic reticulum (ER) (Scott et al., 2022; Martuscello et al., 2023). This mechanism is now looked at as a new possible therapeutic target for ET.
In the field of tic disorders, the newly published criteria for the diagnosis of functional tic-like behavior disorders by a panel of experts is contributing to better defining this condition that has greatly affected the early adolescent population since the recent COVID-19 pandemic (Pringsheim et al., 2023).
Successful clinical trials have showed long-lasting benefit (up to 144 weeks) of Omaveloxolone, a Nrf2 activator, for the treatment of Friedreich's ataxia; sodium oxybate, the sodium salt of γ-hydroxybutyrate, for ET of voice with normalization of impaired brain circuits; and Ecopipam, a dopamine antagonist, for Tourette syndrome (Lynch et a., 2023; O’Flynn et al., 2023; Gilbert et al., 2023). On the other side, the characterization of a larger cohort of patients with Caspr2-realted conditions described new phenotypes (paroxysmal orthostatic and segmental myoclonus and paroxysmal ataxia) worth detecting, as they represent treatable conditions with established immune-modulator approaches (i.e. intravenous immunoglobulin) (Gövert et al., 2023).
Finally, the last year has also seen approval by the Food and Drug Administration (FDA) of Valbenazine for HD, long-lasting botulinum toxin (DaxibotulinumtoxinA) for cervical dystonia, and of staged bilateral MRI-guided focused ultrasound (MRgFUS) for ET.
References - Hypokinetic:
- Siderowf A, Concha-Marambio L, Lafontant DE, et al. Assessment of heterogeneity among participants in the Parkinson's Progression Markers Initiative cohort using α-synuclein seed amplification: a cross-sectional study. Lancet Neurol. 2023;22(5):407-417.
- Okuzumi A, Hatano T, Matsumoto G, et al. Propagative α-synuclein seeds as serum biomarkers for synucleinopathies. Nat Med. 2023;29(6):1448-1455.
- Vijiaratnam N, Foltynie T. How should we be using biomarkers in trials of disease modification in Parkinson's disease? [published online ahead of print, 2023 Aug 3]. Brain. 2023;awad265. doi:10.1093/brain/awad265
- Okuzumi, A., Hatano, T., Matsumoto, G. et al. Propagative α-synuclein seeds as serum biomarkers for synucleinopathies. Nat Med 29, 1448–1455 (2023). https://doi.org/10.1038/s41591-023-02358-9
- Borsche M, Berg D. Blood-Based α-Synuclein Seeding-A New Era for Identifying Parkinsonian Syndromes. Mov Disord. 2023;38(8):1397-1398. doi:10.1002/mds.29555
- Wattjes MP, Huppertz HJ, Mahmoudi N, et al. Brain MRI in Progressive Supranuclear Palsy with Richardson's Syndrome and Variant Phenotypes [published online ahead of print, 2023 Aug 6]. Mov Disord. 2023;10.1002/mds.29527.
- Oura, K., Yamaguchi, T., Nozaki, R., Taguchi, K., Suzuki, Y., Takahashi, K., Takahashi, K., Iwaoka, K., Takahashi, M., Itabashi, R. and Maeda, T. (2023), Vagus Nerve Ultrasonography Helps Distinguish Multiple System Atrophy from Other Parkinsonian Syndromes. Mov Disord Clin Pract. https://doi.org/10.1002/mdc3.13859
- Darricau M, Katsinelos T, Raschella F, et al. Tau seeds from patients induce progressive supranuclear palsy pathology and symptoms in primates. Brain. 2023;146(6):2524-2534. doi:10.1093/brain/awac428
- Giladi N, Alcalay RN, Cutter G, et al. Safety and efficacy of venglustat in GBA1-associated Parkinson's disease: an international, multicentre, double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2023;22(8):661-671. doi:10.1016/S1474-4422(23)00205-3
- Jennings D, Huntwork-Rodriguez S, Vissers MFJM, et al. LRRK2 Inhibition by BIIB122 in Healthy Participants and Patients with Parkinson's Disease. Mov Disord. 2023;38(3):386-398. doi:10.1002/mds.29297
- Hauser RA, Espay AJ, Ellenbogen AL, et al. IPX203 vs Immediate-Release Carbidopa-Levodopa for the Treatment of Motor Fluctuations in Parkinson Disease: The RISE-PD Randomized Clinical Trial [published online ahead of print, 2023 Aug 14]. JAMA Neurol.
- Virameteekul S, Revesz T, Jaunmuktane Z, Warner TT, De Pablo-Fernández E. Clinical Diagnostic Accuracy of Parkinson's Disease: Where Do We Stand?. Mov Disord. 2023;38(4):558-566. doi:10.1002/mds.29317
- Joza S, Hu MT, Jung KY, et al. Progression of clinical markers in prodromal Parkinson's disease and dementia with Lewy bodies: a multicentre study. Brain. 2023;146(8):3258-3272. doi:10.1093/brain/awad072
- Street D, Jabbari E, Costantini A, et al. Progression of atypical parkinsonian syndromes: PROSPECT-M-UK study implications for clinical trials. Brain. 2023;146(8):3232-3242. doi:10.1093/brain/awad105
- Lanore A, Casse F, Tesson C, et al. Differences in Survival across Monogenic Forms of Parkinson's Disease. Ann Neurol. 2023;94(1):123-132. doi:10.1002/ana.26636
- Eschlboeck S, Goebel G, Eckhardt C, et al. Development and Validation of a Prognostic Model to Predict Overall Survival in Multiple System Atrophy. Mov Disord Clin Pract. 2023;10(9):1368-1376. Published 2023 Jul 17. doi:10.1002/mdc3.13822
- Park KW, Wu HJ, Yu T, Mahal R, Mirian MS, McKeown MJ. Potential Pitfalls of Remote and Automated Video Assessments of Movements Disorders. Mov Disord. 2023;38(3):504-506. doi:10.1002/mds.29325
References - Hypokinetic:
- Harrer P, Škorvánek M, Kittke V, Dzinovic I, Borngräber F, Thomsen M, Mandel V, Svorenova T, Ostrozovicova M, Kulcsarova K, Berutti R, Busch H, Ott F, Kopajtich R, Prokisch H, Kumar KR, Mencacci NE, Kurian MA, Di Fonzo A, Boesch S, Kühn AA, Blümlein U, Lohmann K, Haslinger B, Weise D, Jech R, Winkelmann J, Zech M. Dystonia Linked to EIF4A2 Haploinsufficiency: A Disorder of Protein Translation Dysfunction. Mov Disord. 2023 Jul 23. doi: 10.1002/mds.29562. Epub ahead of print. PMID: 37485550.
- Nasca A, Mencacci NE, Invernizzi F, Zech M, Keller Sarmiento IJ, Legati A, Frascarelli C, Bustos BI, Romito LM, Krainc D, Winkelmann J, Carecchio M, Nardocci N, Zorzi G, Prokisch H, Lubbe SJ, Garavaglia B, Ghezzi D. Variants in ATP5F1B are associated with dominantly inherited dystonia. Brain. 2023 Jul 3;146(7):2730-2738. doi: 10.1093/brain/awad068. PMID: 36860166; PMCID: PMC10316767.
- Monfrini E, Ciolfi A, Cavallieri F, Ferilli M, Soliveri P, Pedace L, Erro R, Del Sorbo F, Valzania F, Fioravanti V, Cossu G, Pellegrini M, Salviati L, Invernizzi F, Oppo V, Murgia D, Giometto B, Picillo M, Garavaglia B, Morgante F, Tartaglia M, Carecchio M, Di Fonzo A. Adult-onset KMT2B-related dystonia. Brain Commun. 2022 Oct 26;4(6):fcac276. doi: 10.1093/braincomms/fcac276. PMID: 36483457; PMCID: PMC9724767.
- Krenn M, Sommer R, Sycha T, Zech M. GNAO1 Haploinsufficiency Associated with a Mild Delayed-Onset Dystonia Phenotype. Mov Disord. 2022 Dec;37(12):2464-2466. doi: 10.1002/mds.29258. Epub 2022 Oct 23. PMID: 36273395.
- Wirth T, Garone G, Kurian MA, Piton A, Roze E, Lin JP, Tranchant C, Cif L, Doummar D, Anheim M. Reply to: "GNAO1 Haploinsufficiency Associated with a Mild Delayed-Onset Dystonia Phenotype". Mov Disord. 2022 Dec;37(12):2466-2467. doi: 10.1002/mds.29256. PMID: 36533587.
- Dzinovic I, Winkelmann J, Zech M. Genetic intersection between dystonia and neurodevelopmental disorders: Insights from genomic sequencing. Parkinsonism Relat Disord. 2022 Sep;102:131-140. doi: 10.1016/j.parkreldis.2022.08.019. Epub 2022 Aug 28. PMID: 36088199.
- Erro R, Monfrini E, Di Fonzo A. Early-onset inherited dystonias versus late-onset idiopathic dystonias: Same or different biological mechanisms? Int Rev Neurobiol. 2023;169:329-346. doi: 10.1016/bs.irn.2023.05.002. Epub 2023 Jun 27. PMID: 37482397.
- Tsagkaris S, Yau EKC, McClelland V, Papandreou A, Siddiqui A, Lumsden DE, Kaminska M, Guedj E, Hammers A, Lin JP. Metabolic patterns in brain 18F-fluorodeoxyglucose PET relate to aetiology in paediatric dystonia. Brain. 2023 Jun 1;146(6):2512-2523. doi: 10.1093/brain/awac439. PMID: 36445406; PMCID: PMC10232264.
- Pavelekova P, Necpal J, Jech R, Havrankova P, Svantnerova J, Jurkova V, Gdovinova Z, Lackova A, Han V, Winkelmann J, Zech M, Skorvanek M. Predictors of whole exome sequencing in dystonic cerebral palsy and cerebral palsy-like disorders. Parkinsonism Relat Disord. 2023 Jun;111:105352. doi: 10.1016/j.parkreldis.2023.105352. Epub 2023 Mar 4. PMID: 36997436.
- Wilke C, Pellerin D, Mengel D, Traschütz A, Danzi MC, Dicaire MJ, Neumann M, Lerche H, Bender B, Houlden H; RFC1 study group Stephan Züchner; Schöls L, Brais B, Synofzik M. GAA-FGF14 ataxia (SCA27B): phenotypic profile, natural history progression and 4-aminopyridine treatment response. Brain. 2023 May 11:awad157. doi: 10.1093/brain/awad157. Epub ahead of print. PMID: 37165652.
- Pellerin D, Danzi MC, Wilke C, Renaud M, Fazal S, Dicaire MJ, Scriba CK, Ashton C, Yanick C, Beijer D, Rebelo A, Rocca C, Jaunmuktane Z, Sonnen JA, Larivière R, Genís D, Molina Porcel L, Choquet K, Sakalla R, Provost S, Robertson R, Allard-Chamard X, Tétreault M, Reiling SJ, Nagy S, Nishadham V, Purushottam M, Vengalil S, Bardhan M, Nalini A, Chen Z, Mathieu J, Massie R, Chalk CH, Lafontaine AL, Evoy F, Rioux MF, Ragoussis J, Boycott KM, Dubé MP, Duquette A, Houlden H, Ravenscroft G, Laing NG, Lamont PJ, Saporta MA, Schüle R, Schöls L, La Piana R, Synofzik M, Zuchner S, Brais B. Deep Intronic FGF14 GAA Repeat Expansion in Late-Onset Cerebellar Ataxia. N Engl J Med. 2023 Jan 12;388(2):128-141. doi: 10.1056/NEJMoa2207406. Epub 2022 Dec 14. PMID: 36516086; PMCID: PMC10042577.
- Maroilley T, Tsai MH, Mascarenhas R, Diao C, Khanbabaei M, Kaya S, Depienne C, Tarailo-Graovac M, Klein KM. A novel FAME1 repeat configuration in a European family identified using a combined genomics approach. Epilepsia Open. 2023 Jun;8(2):659-665. doi: 10.1002/epi4.12702. Epub 2023 Feb 16. PMID: 36740228; PMCID: PMC10235570.
- Braz BY, Wennagel D, Ratié L, de Souza DAR, Deloulme JC, Barbier EL, Buisson A, Lanté F, Humbert S. Treating early postnatal circuit defect delays Huntington's disease onset and pathology in mice. Science. 2022 Sep 23;377(6613):eabq5011. doi: 10.1126/science.abq5011. Epub 2022 Sep 23. PMID: 36137051.
- Schultz JL, Langbehn DR, Al-Kaylani HM, van der Plas E, Koscik TR, Epping EA, Espe-Pfeifer PB, Martin EP, Moser DJ, Magnotta VA, Nopoulos PC. Longitudinal Clinical and Biological Characteristics in Juvenile-Onset Huntington's Disease. Mov Disord. 2023 Jan;38(1):113-122. doi: 10.1002/mds.29251. Epub 2022 Nov 1. PMID: 36318082; PMCID: PMC9851979.
- Maas RPPWM. Preparing for Disease-Modification Trials in Degenerative Cerebellar Ataxias: Which Endpoints to Choose? Mov Disord. 2023 Jun;38(6):917-923. doi: 10.1002/mds.29388. PMID: 37475615.
- Coarelli G, Coutelier M, Durr A. Autosomal dominant cerebellar ataxias: new genes and progress towards treatments. Lancet Neurol. 2023 Aug;22(8):735-749. doi: 10.1016/S1474-4422(23)00068-6. PMID: 37479376.
- Feigin A, Evans EE, Fisher TL, Leonard JE, Smith ES, Reader A, Mishra V, Manber R, Walters KA, Kowarski L, Oakes D, Siemers E, Kieburtz KD, Zauderer M; Huntington Study Group SIGNAL investigators. Pepinemab antibody blockade of SEMA4D in early Huntington's disease: a randomized, placebo-controlled, phase 2 trial. Nat Med. 2022 Oct;28(10):2183-2193. doi: 10.1038/s41591-022-01919-8. Epub 2022 Aug 8. Erratum in: Nat Med. 2022 Oct 4;: PMID: 35941373; PMCID: PMC9361919.
- Scott L, Puryear CB, Belfort GM, Raines S, Hughes ZA, Matthews LG, Ravina B, Wittmann M. Translational Pharmacology of PRAX-944, a Novel T-Type Calcium Channel Blocker in Development for the Treatment of Essential Tremor. Mov Disord. 2022 Jun;37(6):1193-1201. doi: 10.1002/mds.28969. Epub 2022 Mar 7. PMID: 35257414; PMCID: PMC9310641.
- Martuscello RT, Chen ML, Reiken S, Sittenfeld LR, Ruff DS, Ni CL, Lin CC, Pan MK, Louis ED, Marks AR, Kuo SH, Faust PL. Defective cerebellar ryanodine receptor type 1 and endoplasmic reticulum calcium 'leak' in tremor pathophysiology. Acta Neuropathol. 2023 Aug;146(2):301-318. doi: 10.1007/s00401-023-02602-z. Epub 2023 Jun 19. PMID: 37335342; PMCID: PMC10350926.
- Pringsheim T, Ganos C, Nilles C, Cavanna AE, Gilbert DL, Greenberg E, Hartmann A, Hedderly T, Heyman I, Liang H, Malaty I, Malik O, Debes NM, Vahl KM, Munchau A, Murphy T, Nagy P, Owen T, Rizzo R, Skov L, Stern J, Szejko N, Worbe Y, Martino D. European Society for the Study of Tourette Syndrome 2022 criteria for clinical diagnosis of functional tic-like behaviours: International consensus from experts in tic disorders. Eur J Neurol. 2023 Apr;30(4):902-910. doi: 10.1111/ene.15672. Epub 2023 Jan 13. PMID: 36587367.
- Lynch DR, Chin MP, Boesch S, Delatycki MB, Giunti P, Goldsberry A, Hoyle JC, Mariotti C, Mathews KD, Nachbauer W, O'Grady M, Perlman S, Subramony SH, Wilmot G, Zesiewicz T, Meyer CJ. Efficacy of Omaveloxolone in Friedreich's Ataxia: Delayed-Start Analysis of the MOXIe Extension. Mov Disord. 2023 Feb;38(2):313-320. doi: 10.1002/mds.29286. Epub 2022 Nov 29. PMID: 36444905.
- O'Flynn LC, Frucht SJ, Simonyan K. Sodium Oxybate in Alcohol-Responsive Essential Tremor of Voice: An Open-Label Phase II Study. Mov Disord. 2023 Jul 14. doi: 10.1002/mds.29529. Epub ahead of print. PMID: 37448353.
- Gilbert DL, Dubow JS, Cunniff TM, Wanaski SP, Atkinson SD, Mahableshwarkar AR. Ecopipam for Tourette Syndrome: A Randomized Trial. Pediatrics. 2023 Feb 1;151(2):e2022059574. doi: 10.1542/peds.2022-059574. PMID: 36628546.
- Gövert F, Abrante L, Becktepe J, Balint B, Ganos C, Hofstadt-van Oy U, Krogias C, Varley J, Irani SR, Paneva S, Titulaer MJ, de Vries JM, Boon AJW, Schreurs MWJ, Joubert B, Honnorat J, Vogrig A, Ariño H, Sabater L, Dalmau J, Scotton S, Jacob S, Melzer N, Bien CG, Geis C, Lewerenz J, Prüss H, Wandinger KP, Deuschl G, Leypoldt F. Distinct movement disorders in contactin-associated-protein-like-2 antibody-associated autoimmune encephalitis. Brain. 2023 Feb 13;146(2):657-667. doi: 10.1093/brain/awac276. PMID: 35875984.
Read more Moving Along:






